0 引言
高温聚合物电解质膜燃料电池(HT-PEMFC)是一种在120~200 ℃运行的燃料电池,对氢源中一氧化碳(CO)的耐受度可达3%~5%[1-2]。目前,制氢主要原料是天然气和石油,利用生物质制氢可减少碳排放。然而生物质收集困难、运输成本较高,且热值较低。将生物质制备为生物油,储存和运输更加方便。生物油黏稠、成分复杂,生物油水相中含有乙酸、丙酮、乙二醇等轻组分,适合重整制氢[3]。关于生物油水蒸气重整制氢,有对生物油单一模型化合物进行制氢的模拟研究[4-6]、催化实验研究[7-9]以及Nabgan等[10]对生物油混合物的实验研究。生物油制氢得到适合燃料电池应用的低CO优化工艺研究还有待开展。
生物油制氢可为燃料电池提供氢源,分析适合燃料电池用氢需要的高氢低CO生物油制氢优化工艺有重要意义。本文以一定比例混合物模拟生物油轻组分,对其进行水蒸气重整制氢高氢低CO工艺研究。首先对生物油水蒸气重整制氢反应进行热力学计算,然后利用Design-Expert软件的响应面分析法[11],以最大H2产率和CO干基摩尔浓度在3%以下为优化条件,确定适合燃料电池应用的低CO生物油水蒸气重整制氢最优条件。
1 生物油重整制氢反应及分析方法
1.1 模拟生物油水蒸气重整制氢反应
生物油组分复杂,含有醇、酯、酮、酸、醛及酚类物质。参考文献[12]取定模拟生物油(主要为轻组分)的组成:每1 mol模拟生物油中含有乙醇0.110 mol、乙酸甲酯0.021 mol、羟基丙酮0.321 mol、乙酸0.450 mol、糠醛0.063 mol、邻甲氧基苯酚0.035 mol,模拟生物油分子式为C2.706H5.044O1.890。生物油重整制氢的总反应为
C2.706H5.044O1.890+3.522H2O → 6.044H2 +
2.706CO2。
(1)
在反应体系中除有制氢反应外,还有水汽变换反应、甲烷化反应及积炭生成反应等。
1.2 分析方法
(1)吉布斯自由能最小化法。应用Aspen Plus分析软件中RGibbs反应器,选择PR-BM物性方法,并进行以下假设:①假设反应器处于恒温恒压状态;②假设反应达到相平衡和化学反应平衡;③反应只考虑主要产物CH4、H2、CO、CO2、C(s)等。
(2)响应面分析法(RSM)。应用Design-Expert软件分析两级自变量(温度T、压力p、水碳比S/C (水与生物油中碳的摩尔数之比))对生物油水蒸气重整产物组成的影响,对数据进行统计计算,拟合自变量与因变量之间的关系式,并分析统计结果。
1.3 分析参数定义
生物油平衡转化率:
(2)
H2产率:
(3)
CO干基摩尔浓度:
(4)
此外,CO2、CH4干基摩尔浓度FCO2、FCH4定义与式(4)类似。
2 结果与讨论
2.1 反应条件对生物油重整制氢的影响
在反应温度300~1 500 K、压力0.1~0.7 MPa和水碳比1.0~7.0条件下,计算了生物油水蒸气重整反应,得到的生物油平衡转化率接近100%。图1为反应条件对生物油重整制氢反应的影响。
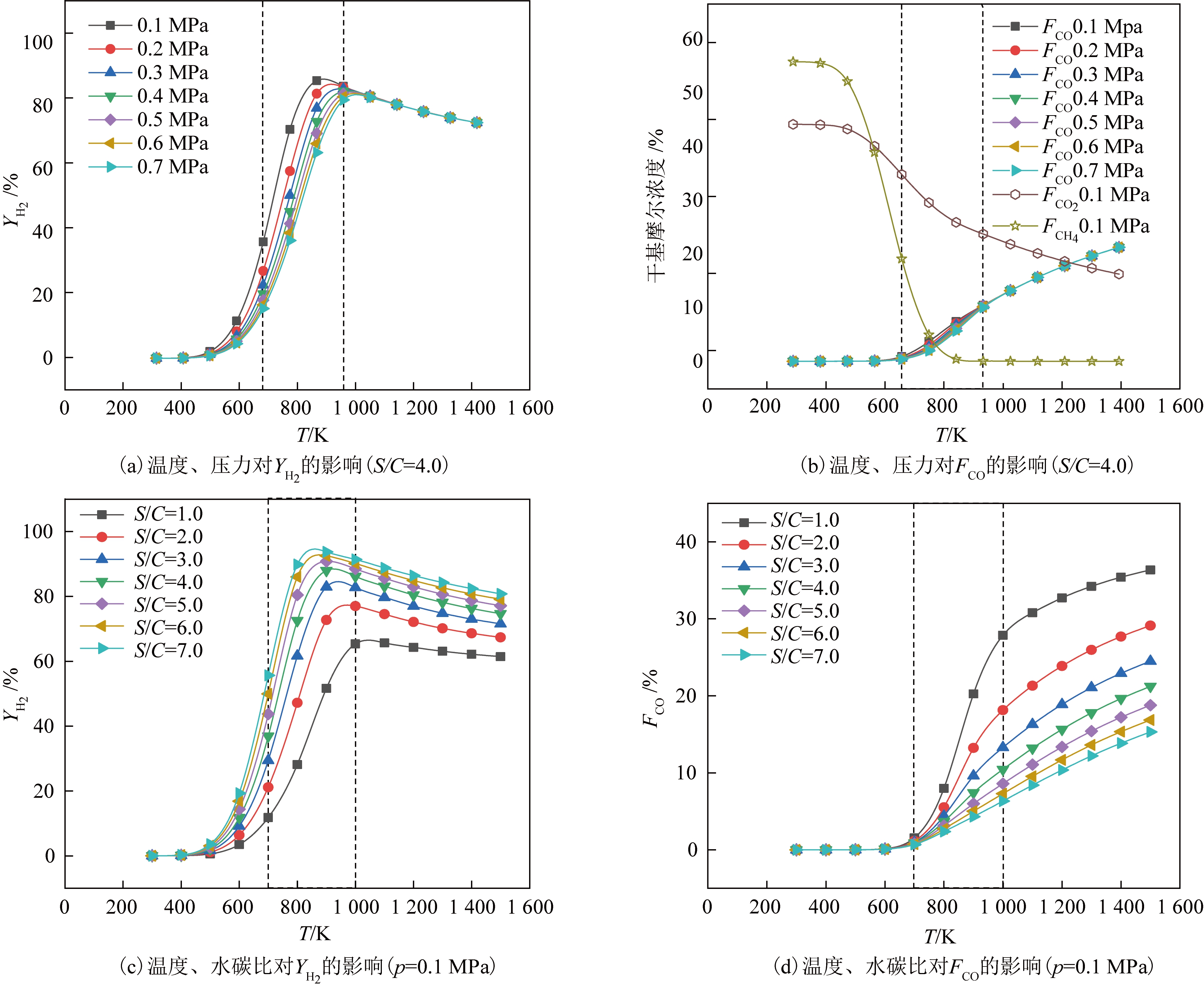
图1 反应条件对生物油重整制氢反应的影响
Figure 1 Effect of reaction conditions on hydrogen production from bio-oil reforming
由图1(a)可知,YH2随着温度的升高先增大后减小,在800~1 200 K时YH2达到最大。由于生物油重整制氢是吸热反应,温度升高有利于反应进行;在900 K以后YH2随温度升高又有所降低,主要是由于发生了逆水汽变换反应。压力对YH2的影响主要体现在600~1 000 K,低压0.1 MPa下有最高的YH2,主要是由于生物油制氢总反应是体积增大的反应。由图1(b)可知,在600~1 200 K随着温度升高,FCO增加;压力对FCO的影响主要体现在700~1 000 K,提高压力可稍降低FCO的数值,但不显著。FCO2在考察范围内随温度升高而逐渐降低;FCH4在400~900 K随温度升高降至零,这是由于较高温度下甲烷发生了水蒸气重整反应。由图1(c)可知,YH2随水碳比增大而增大,且随着水碳比的增大,YH2的峰值增大并向低温方向移动。由图1(d)可知,FCO随温度的升高和水碳比的降低而增加。由图1结果可知,要得到较大YH2及较小FCO,可取温度700~1 000 K和水碳比4.0~6.0及低压下进行响应面分析,在此范围内无积碳产生。过高水碳比会增加生产热负荷,因此将水碳比的上限设置为6.0。
2.2 响应面分析及结果优化
2.2.1 响应面实验方案与回归模型
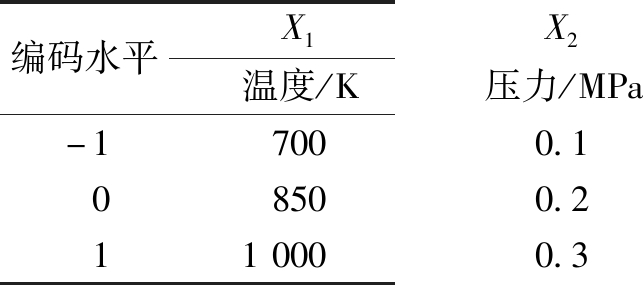
根据上述结果,在较高YH2范围内进行分析设计,响应面法实验变量编码及水平列于表1;将响应面分析需要的15组反应条件及吉布斯模型得到的相应条件热力学结果列于表2。
表1 响应面法实验变量编码及水平
Table 1 Codes and levels of experimental variables in response surface methodology
编码水平X1X2X3温度/K压力/MPa水碳比-17000.14.008500.25.0110000.36.0
表2 Design-Expert统计计算数据
Table 2 Statistical calculation data of Design-Expert
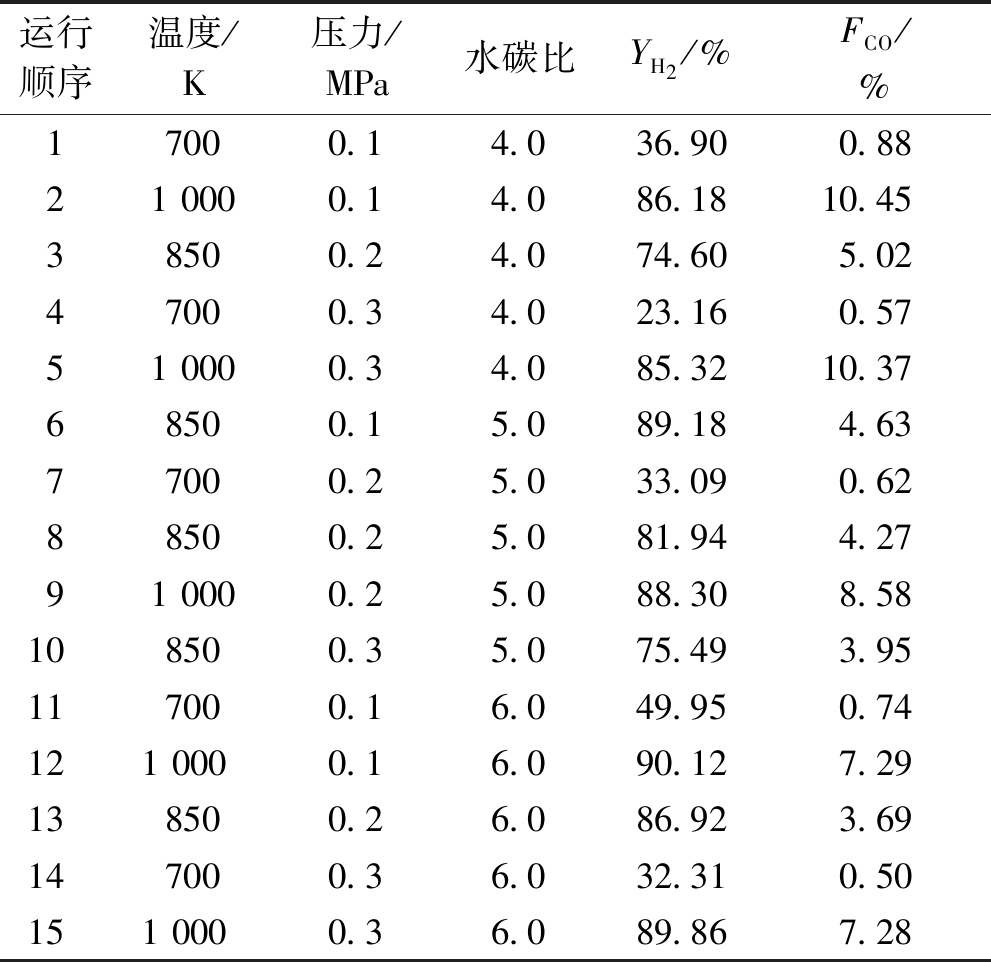
运行顺序温度/K压力/MPa水碳比YH2/%FCO/%17000.14.036.900.88210000.14.086.1810.4538500.24.074.605.0247000.34.023.160.57510000.34.085.3210.3768500.15.089.184.6377000.25.033.090.6288500.25.081.944.27910000.25.088.308.58108500.35.075.493.95117000.16.049.950.741210000.16.090.127.29138500.26.086.923.69147000.36.032.310.501510000.36.089.867.28
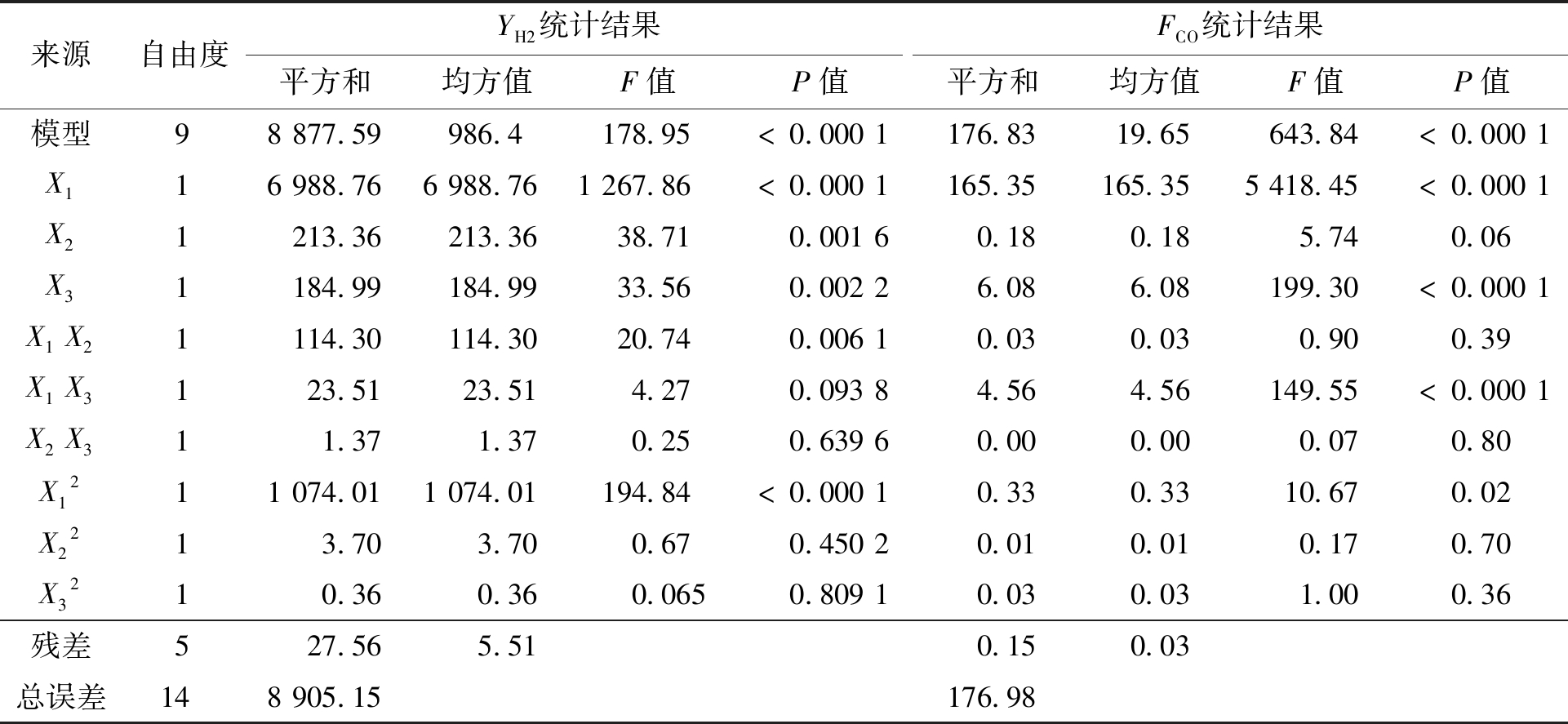
将参数YH2、FCO进行以温度、压力、水碳比为自变量的二次回归拟合,得到模型公式分别为式(5)、(6),其方差分析统计结果见表3。
YH2=81.30 +26.44X1 -4.62 X2 +4.30 X3 +
3.78 X1X2 -1.71 X1X3-0.41 X2X3-
20.44 X12+1.20 X22-0.37 X32;
(5)
FCO=4.25 +4.07 X1 -0.13 X2 -0.78 X3 +
0.06 X1X2-0.76 X1X3 +0.02 X2X3-
0.36 X12 -0.04 X22 +0.11 X32。
(6)
式中:X1、X2、X3均为各项“归一值”。式(5)、(6)的回归拟合系数R12、R22 均为0.99。在YH2、FCO残差正态分布图上,散点分布基本趋于一条直线,表明回归模型拟合效果较好。
表3中YH2、FCO回归模型的F值分别为178.95、643.84,P值均<0.000 1,说明模型(5)、(6)极显著。对于YH2,参数X1及二次项X12的影响极显著(P<0.000 1);3个自变量中,X1对YH2
表3 YH2和FCO的方差分析统计结果
Table 3 Statistical results of variance analysis of YH2 and FCO
来源自由度YH2统计结果FCO统计结果平方和均方值F值P值平方和均方值F值P值模型98877.59986.4178.95<0.0001176.8319.65643.84<0.0001X116988.766988.761267.86<0.0001165.35165.355418.45<0.0001X21213.36213.3638.710.00160.180.185.740.06X31184.99184.9933.560.00226.086.08199.30<0.0001X1X21114.30114.3020.740.00610.030.030.900.39X1X3123.5123.514.270.09384.564.56149.55<0.0001X2X311.371.370.250.63960.000.000.070.80X1211074.011074.01194.84<0.00010.330.3310.670.02X2213.703.700.670.45020.010.010.170.70X3210.360.360.0650.80910.030.031.000.36残差527.565.510.150.03总误差148905.15176.98
的影响最大,其次是X2和X3,因此应主要合理控制温度范围以获得高YH2。对于FCO,参数X1、X3及二次项X1X3的影响极显著(P<0.000 1);3个自变量中,X1和X3对FCO的影响大,因此应主要控制温度和水碳比的范围以获得低FCO。
2.2.2 变量的交互作用
根据图1结果,取温度700~1 000 K和压力0.1~0.3 MPa进行分析,得到水碳比为4.0时压力和温度对YH2和FCO的交互影响如图2所示。由图2可知,在700~1 000 K时,随温度升高,YH2先增加后稍降低,FCO持续增加;低压下YH2数值较高,降低压力可稍降低FCO的数值,但不显著。由此可知,温度是影响FCO的主要因素,而压力影响较小。水碳比为4.0时,要得到较低的FCO,至少将温度控制在880 K以下。
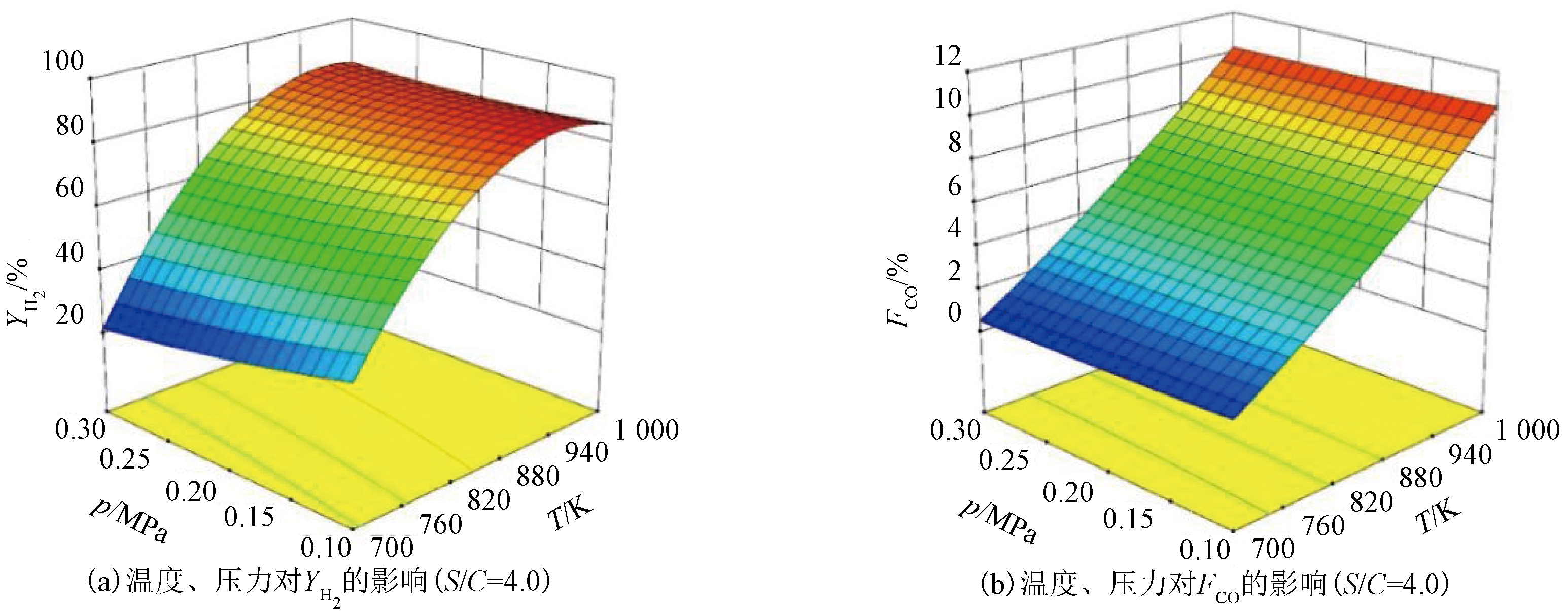
图2 温度和压力对YH2和FCO的交互影响
Figure 2 Interaction effects of temperature and pressure on YH2 and FCO
根据图1结果,取温度700~1 000 K和水碳比4.0~6.0进行分析,得到0.1 MPa时温度和水碳比对YH2和FCO的交互影响如图3所示。由图3可知,YH2随温度升高先增大后减小,YH2随水碳比增加而增大;FCO随温度的升高和水碳比的降低而增大。得到较高YH2和较低FCO的温度在820 K附近。
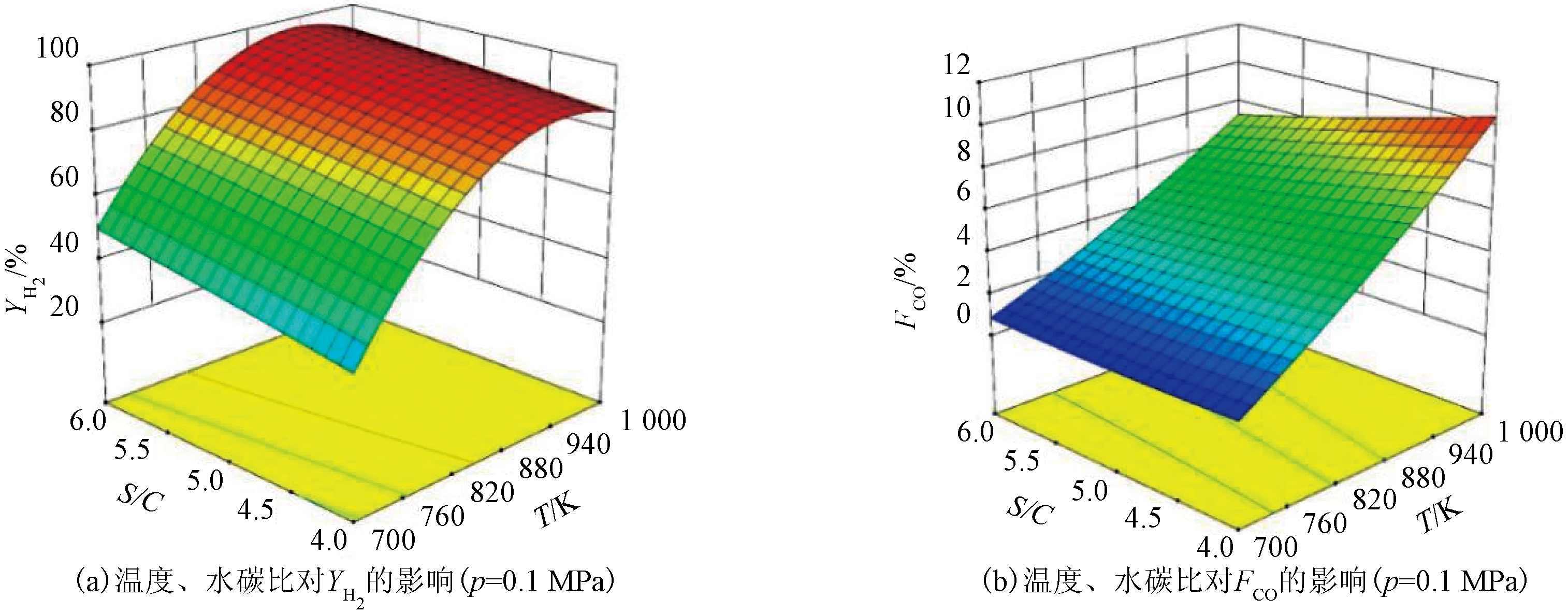
图3 温度和水碳比对YH2和FCO的交互影响
Figure 3 Interaction effects of temperature and water-carbon ratio on YH2 and FCO
2.2.3 工艺数值优化与检验
应用RSM方法确定生物油水蒸气重整制氢高氢低CO的最佳工艺条件。优化约束条件:①YH2最大;②FCO达到3%及以下。得到较优及典型的3个响应面优化结果列于表4,可信度均在0.92以上。表4还列出了优化工艺数据与生物油水蒸气重整制氢实验结果[13-14]对比,其中文献[14]的对比数据是反应中不添加氧的情况,对比结果接近,证明此优化结果可信任。运用吉布斯反应器模型,对响应面优化条件进行生物油水蒸气重整模拟检验,得到反应平衡产物,并将其与统计拟合的YH2和FCO进行对比,得到表5优化数据验证结果。经过检验,响应面预测结果YH2、FCO与Aspen Plus模拟数据的相对误差均在5%以内。在温度为814.98 K、压力为0.10 MPa、水碳比为6.00时,有最高的H2产率(YH2) 88.74%和较低的CO干基摩尔浓度(FCO) 3.07%。
表4 响应面优化结果及对比
Table 4 Response surface optimization results and comparison
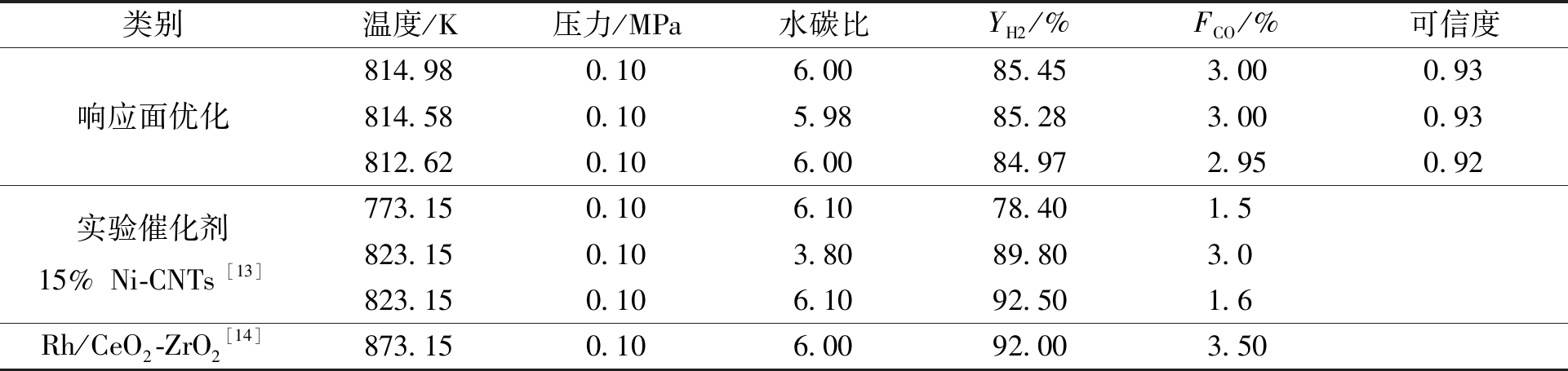
类别温度/K压力/MPa水碳比YH2/%FCO/%可信度响应面优化实验催化剂15%Ni-CNTs[13]Rh/CeO2-ZrO2[14]814.980.106.0085.453.000.93814.580.105.9885.283.000.93812.620.106.0084.972.950.92773.150.106.1078.401.5823.150.103.8089.803.0823.150.106.1092.501.6873.150.106.0092.003.50
表5 优化数据验证结果
Table 5 Optimized data validation results
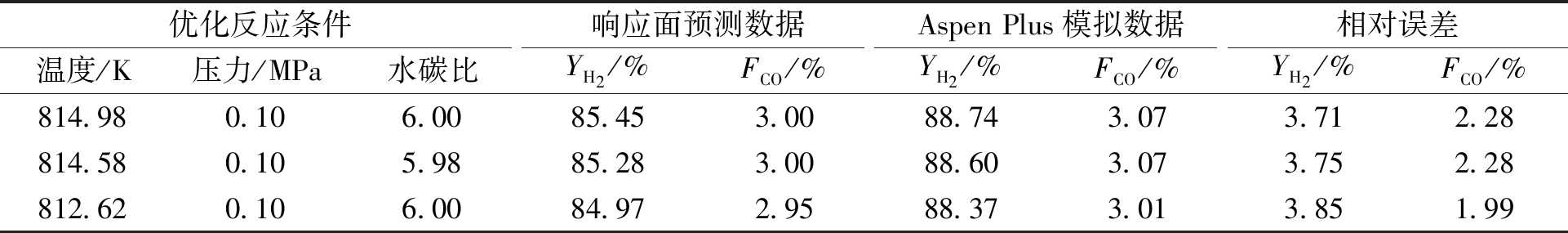
优化反应条件响应面预测数据AspenPlus模拟数据相对误差温度/K压力/MPa水碳比YH2/%FCO/%YH2/%FCO/%YH2/%FCO/%814.980.106.0085.453.0088.743.073.712.28814.580.105.9885.283.0088.603.073.752.28812.620.106.0084.972.9588.373.013.851.99
3 结论
采用Aspen Plus分析软件并结合响应面法,对基于燃料电池应用需求的生物油水蒸气重整过程进行了热力学分析。以H2产率和CO干基摩尔浓度为优化指标,得到了适用于高温聚合物电解质膜燃料电池的生物油水蒸气重整制氢优化工艺条件,并与相关实验结果进行了对比。
(1)较高温度、低压及高水碳比有利于提高H2产率。H2产率的峰值出现在800~1 200 K之间;压力的增大会抑制H2产生,0.1 MPa下有最高的H2产率;水碳比的增大会提高H2产率,且水碳比增大会使H2产率峰值向低温方向移动。
(2)低温、较高压力和高水碳比有利于降低CO干基摩尔浓度。CO干基摩尔浓度随着温度的升高而增加,提高压力和水碳比会抑制CO产生;压力对CO干基摩尔浓度影响较小,压力对CO干基摩尔浓度影响较大的温度在700~1 000 K之间。
(3)适合燃料电池应用的生物油水蒸气重整制氢低CO最佳反应条件为温度814.98 K、压力0.10 MPa、水碳比6.00,此时热力学上有最高H2产率88.74%和较低CO干基摩尔浓度3.07%。将统计优化数据与热力学计算及文献实验结果进行了对比,优化结果得到了验证。
[1] ELLAMLA H R,BUJLO P,SITA C,et al.Comparatative analysis on various reformers supplied with different fuels and integrated with high temperature PEM fuel cells[J].Chemical engineering science,2016,154:90-99.
[2] V ZQUEZ F V,SIMELL P,PENNANEN J,et al.Reactor design and catalysts testing for hydrogen production by methanol steam reforming for fuel cells applications[J].International journal of hydrogen energy,2016,41(2):924-935.
ZQUEZ F V,SIMELL P,PENNANEN J,et al.Reactor design and catalysts testing for hydrogen production by methanol steam reforming for fuel cells applications[J].International journal of hydrogen energy,2016,41(2):924-935.
[3] 张方柏.生物质油催化重整制氢用镍基催化剂研究[D].成都:成都理工大学,2014.
ZHANG F B.Catalytic reforming of bio-oil for hydrogen production over Ni-based catalysts[D].Chengdu:Chengdu University of Technology,2014.
[4] 包秀秀.生物油轻质组分模型化合物重整制氢研究[D].杭州:浙江大学,2015.
BAO X X.Research about the steam reforming of model compound of bio-oil light component for hydrogen production[D].Hangzhou:Zhejiang University,2015.
[5] 安森萌,付鹏,易维明.乙酸水蒸气重整制氢反应的热力学分析[J].太阳能学报,2013,34(9):1526-1530.
AN S M,FU P,YI W M.Thermodynamic analysis of hydrogen production via steam reforming of acetic acid[J].Acta energiae solaris sinica,2013,34(9):1526-1530.
[6] 王东旭,肖显斌,李文艳.乙酸水蒸气重整制氢过程的热力学分析[J].新能源进展,2017,5(5):346-351.
WANG D X,XIAO X B,LI W Y.Thermodynamic analysis on steam reforming of acetic acid for hydrogen production[J].Advances in new and renewable energy,2017,5(5):346-351.
[7] HU X,LU G X.Comparative study of alumina-supported transition metal catalysts for hydrogen generation by steam reforming of acetic acid[J].Applied catalysis B:environmental,2010,99(1/2):289-297.
[8] WANG S R,CAI Q J,ZHANG F,et al.Hydrogen production via catalytic reforming of the bio-oil model compounds:acetic acid,phenol and hydroxyacetone[J].International journal of hydrogen energy,2014,39(32):18675-18687.
[9] 赵星岭.乙二醇低温蒸汽重整制氢的研究[D].烟台:烟台大学,2016.
ZHAO X L.Research on hydrogen production from steam reforming of ethylene glycol at low temperature[D].Yantai:Yantai University,2016.
[10] NABGAN W,TUAN ABDULLAH T A,MAT R,et al.Acetic acid-phenol steam reforming for hydrogen production:effect of different composition of La2O3-Al2O3 support for bimetallic Ni-Co catalyst[J].Journal of environmental chemical engineering,2016,4(3):2765-2773.
[11] 陈俊英,周航宇,唐焕妍,等.响应面法优化纤维素基载体固定糖化酶的研究[J].郑州大学学报(工学版),2019,40(2):66-71.
CHEN J Y,ZHOU H Y,TANG H Y,et al.Optimization for cellulose carrier immobilized glucoamylase by response surface methodology[J].Journal of Zhengzhou university (engineering science),2019,40(2):66-71.
[12] LI W,PAN C Y,ZHANG Q J,et al.Upgrading of low-boiling fraction of bio-oil in supercritical methanol and reaction network[J].Bioresource technology,2011,102(7):4884-4889.
[13] HOU T,YUAN L X,YE T Q,et al.Hydrogen production by low-temperature reforming of organic compounds in bio-oil over a CNT-promoting Ni catalyst[J].International journal of hydrogen energy,2009,34(22):9095-9107.
[14] ARANDIA A,REMIRO A,OAR-ARTETA L,et al.Reaction conditions effect and pathways in the oxidative steam reforming of raw bio-oil on a Rh/CeO2-ZrO2 catalyst in a fluidized bed reactor[J].International journal of hydrogen energy,2017,42(49):29175-29185.